blocked fallopian tube symptoms

Eirmed is an informational platform dedicated to providing reliable, science-based insights on male and female fertility, reproductive health, and natural conception.
Category description for “Medical Articles”
blocked fallopian tube symptoms

Eirmed is an informational platform dedicated to providing reliable, science-based insights on male and female fertility, reproductive health, and natural conception.
Do Prenatal Vitamins Help Get Pregnant? When you decide to start a family, your focus naturally shifts toward health and wellness. You might change your diet, adjust your exercise routine, and start looking into supplements. Among the most discussed are prenatal vitamins. This leads to a common and important question: do prenatal vitamins help you get pregnant? It’s a topic surrounded by a mix of hope, confusion, and misinformation.
This blog post will directly address that question. We will cut through the myths and explore the science behind prenatal vitamins and their role in your conception journey. Whether you are just beginning to think about pregnancy or are actively trying, understanding how these supplements work is a key part of your preparation.
To understand if prenatal vitamins can help with conception, we first need to look at what they are designed to do. A prenatal vitamin is a specially formulated multivitamin that provides the key nutrients an expectant mother and her developing baby need. Their primary purpose is to fill nutritional gaps and support a healthy pregnancy, not necessarily to act as a fertility treatment.
However, the nutrients inside these supplements play a critical role in reproductive health. A body that is well-nourished is better prepared for the demanding process of conception and pregnancy. Let’s look at the key components often found in these fertility supplements and how they contribute to your body’s readiness for pregnancy.

So, let’s get to the core question: will taking a prenatal vitamin make you more fertile? The direct answer is no. Prenatal vitamins are not a magic pill that will increase your chances of getting pregnant overnight. They are not fertility drugs.
However, by creating an optimal nutritional environment in your body, they indirectly support the processes that lead to conception. Think of it like gardening. Tilling the soil and adding nutrients doesn’t guarantee a seed will sprout, but it creates the best possible conditions for it to do so.
Most doctors and fertility specialists recommend that any woman of childbearing age who is considering pregnancy should start taking a prenatal vitamin. The consensus is not that it will act as a fertility aid, but that it is a fundamental step in responsible pregnancy preparation. By addressing potential nutritional deficiencies before you conceive, you improve your overall health, which in turn supports your reproductive system.
If you are trying to conceive, incorporating a prenatal vitamin is a wise and recommended step. Here is some practical advice to help you navigate your options.
This is the most important step. Before you start any new supplement regimen, talk to your healthcare provider. They can:
While prenatal vitamins are not a direct fertility treatment, they are an essential tool for anyone planning a pregnancy. They work by creating the best possible nutritional foundation for conception and ensuring your body is ready to support a healthy baby from the very beginning. The main takeaway is this: taking a prenatal vitamin is a proactive, responsible step that supports your overall and reproductive health.
Instead of viewing them as a way to get pregnant, think of them as a way to prepare for pregnancy. By doing so, you are giving yourself and your future child a healthy head start.
For personalized advice tailored to your health and family goals, consult your healthcare provider to discuss the best path forward for you.

I manage fertility and pregnancy content at EIRMED and work closely with licensed fertility experts to ensure our articles are accurate, science-based, and trustworthy.
A fertility blend for women is a dietary supplement formulated to support female reproductive health and improve the chances of conception. It typically combines:
Herbal extracts (for example, Vitex agnus‑castus, aka Chasteberry)
Amino acids (e.g., L-arginine)
Vitamins & minerals (folic acid, B6, B12, iron, zinc, selenium, vitamin E)
Antioxidants (green tea extract, selenium)
One well-known product is Fertility Blend for women.
According to its manufacturer, the goal is to “improve fertility by optimizing hormonal and menstrual cycle balance”.
When you are trying to get pregnant, the most important pills are not “blends” that promise big changes. They are the simple vitamins needed to keep you and the tiny growing baby safe. The number one helper pill is Folic Acid.
Folic acid (which is Vitamin B9) is the only pill doctors say every single woman who might get pregnant must take.
Folic acid is like a building block for the baby’s body, helping to make new cells and DNA. It is super important because it helps stop serious birth problems with the baby’s brain and spine (called Neural Tube Defects)., These problems happen very, very early—sometimes before a woman even knows she is pregnant.
A good prenatal vitamin is a simple way to make sure you have all the other main vitamins your body needs, like Iron, Calcium, and other B Vitamins. These help keep you strong and energetic while making sure the baby has enough food to grow.

Many fertility blends are mostly collections of vitamins and herbs called “antioxidants.” The idea is that antioxidants help clean up tiny damage inside your body’s cells, including your eggs.
For most women who do not have a specific health problem, the science is not strong enough to say that these blends help them get pregnant faster or easier.
The pills that do have strong scientific proof are usually not for everyone. They are special, high-dose helper pills for women who have been diagnosed with a specific medical problem.
CoQ10 is a powerful energy helper found in every cell. It is like the “powerhouse fuel” for your body. Egg cells need a huge amount of energy to grow big and strong, and this can slow down as women get older.
CoQ10 shows the most benefit for women who are over 35 or who have been told they have Diminished Ovarian Reserve (DOR), meaning they have a low supply of eggs left.
Taking a high dose of CoQ10 before a procedure like IVF is linked to very good results:
Myo-inositol is a natural compound that works by helping the body’s hormones stay balanced. It is often used for women with Polycystic Ovary Syndrome (PCOS).
PCOS causes the body to struggle with hormones, which can stop the woman from releasing an egg (ovulating) regularly. Myo-inositol helps the body listen to its own hormone signals better, which can make the periods more regular and sometimes start ovulation again.
Low levels of Vitamin D are common and have been linked to a higher chance of not being able to conceive.
If a blood test shows your Vitamin D level is too low (under 30 ng/mL), taking a supplement to fix this problem can help. Research suggests that taking Vitamin D can improve the chance of a clinical pregnancy and raise the rate of ovulation, especially for women with PCOS.
Some blends contain herbs, like Vitex agnus-castus (Chaste Tree). Vitex has been studied because it can help normalize a specific hormonal issue where the body makes too much of the hormone prolactin. In these cases, one study found that a supplement containing Vitex helped to normalize progesterone levels and resulted in pregnancy. This suggests it may help if a woman has a luteal phase defect (a problem with the second half of the cycle).
Here are examples of how these scientific findings translate into successful pregnancies:
Cynthia T. shared her story of dealing with PCOS infertility. She said that she started taking a Myo-inositol supplement and quickly noticed her PCOS symptoms getting better. She wrote, “Much to my surprise I’m pregnant now after taking it for only 1 month.” She also planned to keep taking it to manage her PCOS symptoms even after pregnancy, showing that this targeted pill helped solve her specific hormonal problem.
Research focusing on women with low egg reserve (DOR) who were having IVF procedures showed powerful results. In one study, women who used CoQ10 before their treatment saw a significantly higher chance of getting pregnant (28.8% got pregnant compared to 14.1% who did not use it). This evidence proves that CoQ10 gave their eggs the needed energy boost to thrive.
Another woman named Sophia, who also had PCOS, shared her success story after using a Myo-inositol supplement. She said that she used it when she was ready to have her second child and was pregnant within a month. She recommends it to others with PCOS, showing how well this specific supplement can help control hormonal issues when trying to conceive.
Remember that pills that claim to be a “miracle blend” often lack strong scientific proof that they work for everyone. If you are struggling to get pregnant, the best steps are:
This article is for general knowledge only and not medical advice. Always see a healthcare provider for your symptoms or treatments. EIRMED products aid health but do not cure. Results can differ. We use info from public sources, but check with pros for your needs.

Dr. Angela Leung is a reproductive endocrinologist who helps men, women and couples who want to have a baby, preserve their fertility, or just take care of a reproductive health issue. Her focus, besides helping her patients achieve success, is developing a close relationship with them so they feel safe showing vulnerability, confident asking questions and comfortable investing in their fertility journey.
Breakout Ovulation is a common skin concern many women face during their menstrual cycle. Hormonal changes, especially around ovulation, can trigger acne or small pimples, often on the face, chin, or jawline. If you’ve noticed your skin becoming oily or breaking out during this time, you’re not alone — it’s a natural body response linked to fertility hormones.
Understanding why ovulation causes breakouts can help you take control of your skin, manage hormonal balance, and feel more confident every month.
Breakout Ovulation refers to acne or skin flare-ups that appear around the time of ovulation — typically 10 to 14 days before your period. This is the phase when estrogen peaks and luteinizing hormone (LH) triggers the release of an egg.
As your hormones fluctuate, oil glands in your skin become more active, leading to clogged pores and pimples, especially around the chin, jawline, and cheeks. These are the hallmark signs of hormonal acne related to your reproductive cycle.
Yes, ovulation can cause breakouts. During ovulation, your body increases the production of luteinizing hormone (LH) and estrogen, followed by a spike in progesterone. These hormonal shifts can stimulate oil (sebum) production in your skin. When excess oil mixes with dead skin cells, pores get clogged, leading to acne.
In short:
Estrogen increases → giving your skin a natural glow.
LH (luteinizing hormone) spikes → triggering ovulation.
Progesterone and testosterone rise after ovulation → causing oil production to increase.
That extra oil (sebum) can clog pores, allowing bacteria to grow and leading to inflammation — or what we call Breakout Ovulation.
These hormonal changes are completely normal, but the way your skin reacts depends on your genetics, diet, and skincare habits.
Many women notice a face breakout during ovulation, especially around the chin, jawline, and forehead. This is because these areas are most sensitive to hormonal fluctuations.
If you experience breakout ovulation, you might notice:
Pimples on the chin, jawline, or cheeks
Small whiteheads or blackheads around ovulation
Oily skin that feels greasy or sticky
Slight tenderness in acne spots
Acne appearing the same time each month
💡 Tip: Keep your skin clean but not dry. Over washing can trigger even more oil production.
Acne during ovulation is not just a cosmetic issue — it’s a hormonal indicator. When your body prepares to release an egg, androgen hormones rise slightly. These androgens tell your sebaceous glands to make more oil.
Studies show that testosterone and progesterone fluctuations play a key role in ovulation acne. This is why even women with normally clear skin may suddenly notice pimples during their mid-cycle.
Some women experience breakouts before ovulation, about 10–14 days after their last period. This pre-ovulation acne often appears as mild whiteheads or small bumps.
This happens because estrogen starts increasing and your body prepares for the egg release. The hormonal shift can momentarily disrupt skin balance, especially if your skincare routine or diet is inconsistent.
A breakout after ovulation is also common — and in some cases, worse than before. After ovulation, progesterone levels rise to prepare the uterus for possible pregnancy. This hormone also increases sebum production, making pores more likely to clog.
If pregnancy doesn’t occur, hormone levels drop again right before your period, often causing another acne phase.
Yes, in many cases, it can be.
Breakouts around ovulation often indicate that your hormones are active and your body is ovulating normally.
Fertile women commonly experience mild skin changes because of natural estrogen and testosterone fluctuations. So, if you see mild acne around mid-cycle, it’s often a sign that your ovulation cycle is healthy.
However, severe or painful acne may signal hormonal imbalance, PCOS (polycystic ovary syndrome), or other fertility issues — and that’s when professional advice is helpful.
| Hormone | Function | Effect on Skin |
|---|---|---|
| Estrogen | Improves skin texture and hydration | Makes skin look fresh and glowing |
| Progesterone | Increases sebum (oil) production | Can clog pores and cause acne |
| Testosterone | Stimulates oil glands | Triggers pimples, especially around chin and jaw |
| Cortisol | Stress hormone | Can worsen existing acne |
Hormonal breakouts during ovulation are different from typical acne. These pimples are often deeper, red, and painful because they form under the skin.
Appear cyclically (same time every month)
Localized on lower face or neck
Linked to hormonal peaks
🩺 Expert insight from EIRMED:
Hormonal acne is a normal but manageable sign of your body’s reproductive rhythm. Keeping track of your cycle and noting skin changes can help identify patterns — and potential hormonal imbalances if acne becomes severe.
Here are some expert-approved tips to help balance your hormones and protect your skin during ovulation:
Wash your face twice daily with a gentle, non-drying cleanser. Avoid harsh scrubbing; it can worsen inflammation.
Include foods that support hormone balance:
Leafy greens, nuts, and seeds for zinc and magnesium
Omega-3 fatty acids (from fish or flaxseeds)
Avoid high-sugar foods, which spike insulin and worsen acne
High stress raises cortisol, which may increase oil production. Yoga, deep breathing, and proper sleep can regulate hormones naturally.
Water helps flush toxins and balances oil levels. Aim for 8–10 glasses per day.
Topical treatments with salicylic acid or benzoyl peroxide help, but using them excessively can dry and irritate the skin.
If your breakouts are severe, your doctor may recommend:
Hormonal birth control to stabilize estrogen and progesterone
Spironolactone to reduce testosterone effects
Mild retinoids (under medical advice)
Always consult a licensed dermatologist before starting new medications.
Yes, it is completely normal to break out a week after ovulation. This usually happens due to progesterone dominance and decreasing estrogen levels. The oil glands stay active, and your skin may still be purging the effects of mid-cycle hormonal changes.
If the acne persists more than a week or becomes painful, it may be worth checking hormone levels with your doctor to rule out PCOS (Polycystic Ovary Syndrome) or other endocrine issues.
If you experience:
Persistent, painful cystic acne
Acne along with irregular periods or hair growth
Emotional distress from hormonal imbalance
Then, a fertility or hormonal health specialist (like those at EIRMED) can guide you through personalized treatment plans.
A breakout during ovulation refers to a spike in acne (pimples, blackheads, whiteheads, sometimes deeper cysts) that occurs around the mid-point of the menstrual cycle, when the ovary releases an egg.
This is generally driven by hormonal changes impacting the skin’s oil production, pore clogging, inflammation and bacterial activity.
Several hormonal shifts happen around ovulation which affect the skin:
A rise in androgens (for example testosterone) increases sebum (skin oil) production, which can clog pores and lead to acne.
After ovulation, progesterone levels increase and estrogen drops, which can reduce the skin’s normal balancing effect and make pores more prone to blockage and inflammation.
The combination of more oil + more skin cell debris + possible bacteria = higher risk of breakouts.
Typical features include:
Location: the lower face (jawline, chin), sometimes neck and lower cheeks.
Type: could be red, inflamed pimples, cystic lesions (deep under the skin) as well as white-heads or black-heads.
Timing: they often appear mid-cycle (around ovulation) or in the luteal phase (after ovulation) and may follow a pattern each cycle.
Key clues:
It happens consistently each cycle around the same time (mid-cycle or shortly after).
It appears in typical hormonal acne zones (jawline, chin).
You notice increased oiliness or changes in your skin texture around that time.
You may also notice other ovulation symptoms (change in cervical mucus, mild twinge/pain, etc) together.
If your acne timing and pattern match your cycle, hormonal/ovulation-related causes are more likely.
Effective approaches include:
Topical treatments: e.g., salicylic acid (to unclog pores), benzoyl peroxide (to reduce bacteria), retinoids (for cell turnover) — especially helpful when breakout is superficial.
Hormonal treatments: For persistent or severe breakouts, therapies that regulate hormones (birth-control pills with both estrogen + progestin, anti-androgens like spironolactone) may be considered.
Lifestyle & skincare tweaks: Adjust your skincare around the cycle (lighter, non-comedogenic products during high-oil phases), maintain good hygiene, manage stress, eat a balanced diet.
When to see a specialist: If the acne is deep cystic, leaves scars, or is accompanied by signs of hormonal imbalance (irregular periods, excess hair growth, etc) — consult a dermatologist or endocrinologist.
Yes — while you may not be able to stop hormonal shifts entirely, you can reduce their skin-impact:
Use gentle, non-comedogenic skin care especially around ovulation.
Avoid heavy oils or pore-clogging products when skin feels oilier.
Maintain a low-glycemic diet, reduce excess sugars/refined carbs (since insulin spikes can worsen hormonal acne).
Manage stress and sleep well (stress hormones like cortisol can worsen acne).
Track your cycle so you know when your skin might be more vulnerable.
They are related but slightly different:
Pre-menstrual acne (just before your period) is often driven by the luteal phase hormones (high progesterone, dropping estrogen) and shows up ~7-10 days before menstruation.
Ovulation-related breakout occurs earlier (around mid-cycle) and is triggered by the hormone shifts around ovulation itself.
So yes — both fall under “hormonal acne”, but the timing and hormonal triggers differ.
The breakout may last as long as the hormonal trigger persists (i.e., the mid-cycle surge plus the early luteal phase). For many people, the skin returns to baseline after that phase. But if the acne is deep or untreated, it may linger or leave marks/scarring. Consistent treatment and adapting your routine help shorten the duration.
Absolutely. Knowing your cycle and adapting your skincare accordingly gives you a proactive edge. For example:
During ovulation/increased oil phase: use lightweight, oil-control products, gentle exfoliants, avoid heavy moisturisers.
Later when hormones shift: adjust to barrier-supporting care.
Multiple sources suggest that aligning your routine with the menstrual phases helps.
Yes — some underlying hormonal conditions can make breakout patterns worse or more persistent. For example:
Polycystic Ovary Syndrome (PCOS) often features high androgens + irregular cycles + acne.
Conditions causing hormonal imbalance (thyroid disorders etc) may also contribute. If your acne is severe, persistent and accompanies other symptoms (irregular bleeding, hair growth, rapid weight changes), you should consult a healthcare provider.
Understanding Breakout Ovulation helps women feel more confident and informed about their body’s hormonal patterns. By recognizing how and why these skin changes occur, you can take simple steps to support both your fertility and your skin health naturally.
This article is for educational purposes only and not a substitute for professional medical advice. Always consult a licensed healthcare provider before starting any treatment or medication.
Thank you for reading this guide by EIRMED. We hope it helped you understand your ovulation-related skin changes better. Stay connected for more fertility and women’s health insights.

I manage fertility and pregnancy content at EIRMED and work closely with licensed fertility experts to ensure our articles are accurate, science-based, and trustworthy.
Can donating eggs make you infertile?
This is one of the most common fears among women considering egg donation. The short answer is no — egg donation does not cause infertility when done safely by licensed fertility specialists.
At EIRMED, we understand how important fertility health is for every woman, especially when she’s helping another family through egg donation. In this detailed guide, we’ll explain how egg donation works, what happens to your eggs, and why your fertility remains intact after the process.
Egg donation is a medical procedure where a woman donates some of her eggs to help another person or couple conceive through assisted reproductive technology (ART) like IVF.
Every woman is born with 1–2 million eggs, but only about 400–500 of them are released during her lifetime through ovulation. The rest naturally die off with age.
When you donate eggs, the fertility clinic uses hormone medications to help several eggs mature in one cycle — eggs that would otherwise not be used by your body. This means you’re not losing future fertility, you’re just using more eggs in one month instead of over several cycles.
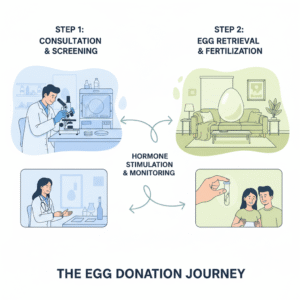 1. Screening and Medical Evaluation
1. Screening and Medical EvaluationBefore donating, the fertility clinic performs a full health check:
Blood tests (hormones, infections)
Ultrasound to check ovarian health
Family and genetic history review
This ensures you’re healthy enough for donation and your future fertility won’t be at risk.
You take fertility medications (like FSH) for about 10–12 days. These medicines help your ovaries grow multiple eggs at once instead of one.
Doctors closely monitor your hormone levels and follicle growth using blood tests and ultrasounds.
When the eggs are ready, you’ll undergo a short, 15–20-minute procedure under mild anesthesia. A needle guided by ultrasound gently removes the mature eggs.
No incisions are made, and recovery is usually quick — most women return to normal activity within 1–2 days.
After retrieval, your ovaries return to normal size within a few weeks. Your body’s natural menstrual and ovulation cycles continue as usual.
The truth is: egg donation does not make you infertile.
Here’s why:
The eggs collected are the ones that would naturally be lost in that cycle.
The process does not damage your ovaries when done correctly.
Your menstrual cycles and ovulation patterns continue normally afterward.
Most egg donors go on to have healthy pregnancies later in life without any issues.
Multiple research studies confirm that egg donation does not reduce fertility or cause early menopause.
A study published in the Fertility and Sterility Journal found that women who donated eggs had no difference in ovarian reserve or fertility outcomes compared to women who did not donate.
Reputable clinics like those listed at EIRMED partner centers follow strict medical protocols approved by organizations such as:
ASRM (American Society for Reproductive Medicine)
ESHRE (European Society of Human Reproduction and Embryology)
While egg donation is safe, some women may experience mild, short-term effects:
Bloating or mild abdominal discomfort
Temporary mood changes
Fatigue after retrieval
Light bleeding or cramping
These usually go away within a few days. Fertility specialists always monitor donors to prevent complications.
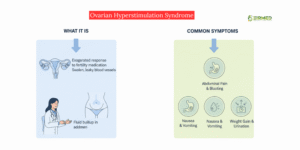
In rare cases (less than 2–5%), women may develop OHSS, a condition where ovaries react strongly to fertility medication.
Symptoms include swelling, nausea, or pain.
Modern clinics minimize this risk by using lower medication doses and close ultrasound monitoring.
If OHSS happens, it’s temporary and treated effectively. It does not lead to long-term infertility.
According to international fertility guidelines, a woman can safely donate eggs up to 6 times in her lifetime.
Each donation is spaced out to allow your body to fully recover. Studies show repeated donations under medical supervision do not reduce egg reserve or future pregnancy chances.
| Myth | Fact |
|---|---|
| Donating eggs uses up all your eggs | You are born with millions; donation uses only a few extra from one cycle |
| Egg retrieval damages ovaries | It’s a minor, safe procedure with no cuts or scarring |
| You’ll have early menopause | Donation does not affect your natural egg reserve |
| You can’t get pregnant later | Thousands of donors have conceived naturally afterward |
Egg donation can be emotionally fulfilling but also sensitive. Some donors worry about the idea of their eggs creating another family. Clinics often offer counseling sessions to ensure you are mentally ready and understand every step of the process.
Choosing a licensed fertility center like EIRMED ensures:
Expert doctors monitor every stage
Safe medication protocols
Low OHSS risk
Ethical donation process
Full protection of your future fertility
Hayley’s story (UK, donor) — “Why I became an egg donor”
Hayley donated eggs three times. She states:
“I did my research and found out it wouldn’t affect my own fertility or my own chances of getting pregnant in future.”
She donated after having her own child and felt comfortable her fertility would remain unaffected. This is a positive example of a donor who felt informed and safe.
Egg donation success for recipients at London Egg Bank — “Sarah and Mark’s Story”
Sarah and Mark had experienced multiple miscarriages and fertility challenges. After using donor eggs, they succeeded in having a healthy baby daughter. They reflect:
“We’ve never looked back after deciding on using an egg donor because without it we wouldn’t have a child right now.”
This demonstrates successful use of donor eggs for recipient families, illustrating positive outcomes.
Donor experience at TFP Fertility — “Alex’s Story”
Alex, age 31, chose to donate eggs and worked through the process aware of what it entailed. She states the donor process as empowering and fulfilling.While not specifically about her future fertility post-donation, it shows a donor who went in informed and comfortable.
“Exposing the dark side of egg donation” — Mercator Net article
The article describes a case where a woman donated eggs (half of her eggs) in exchange for a reduced cost of her own IVF. Later, she was hospitalized, and years afterwards she still struggled with infertility while knowing her donated eggs resulted in another woman’s successful birth.
This story shows that even when a donation process is done, the donor may have long-term emotional or fertility concerns that were not fully addressed.
Reddit donor forum: “Egg donation gone wrong”
One donor at age 24 took part in an egg-donation program due to financial need. She had very low AMH and high FSH (markers of low ovarian reserve) only discovered during donor screening. She ended up feeling hopeless about her own fertility afterwards.
While this is anecdotal, it highlights the importance of counseling, screening, and long-term donor follow-up.
No, donating eggs does not cause infertility in healthy women. The process uses only the eggs that your body would naturally release during that menstrual cycle. Egg retrieval does not reduce your total egg reserve or affect your future fertility if done under medical supervision.
In most cases, no. Egg donors can conceive naturally after donation. The hormones used for stimulation leave the body within a few weeks, and menstrual cycles usually return to normal. However, as with any medical procedure, rare complications (like ovarian hyperstimulation syndrome) can temporarily affect fertility.
According to fertility specialists and the American Society for Reproductive Medicine (ASRM), women can donate eggs up to six times safely, as long as each cycle is properly monitored. Your doctor will evaluate your health and ovarian response before each donation.
Severe complications are extremely rare. During egg retrieval, an ultrasound-guided needle is used to collect eggs from the ovaries. When performed by experienced specialists, it is a low-risk procedure. Any minor discomfort or bloating usually resolves within days.
No long-term side effects have been scientifically linked to fertility medications used in egg donation. These hormones temporarily stimulate the ovaries but do not alter your body’s natural ability to produce eggs in future cycles.
You can safely try to get pregnant after your next normal period, typically 1–2 months after egg donation. Your fertility specialist will confirm when your hormones and ovarian function have returned to baseline.
No, egg donation does not trigger early menopause. Women are born with hundreds of thousands of eggs, and only a small number mature each month. The donation process simply collects the eggs that would otherwise go unused in that cycle.
Some women may feel mild mood changes due to temporary hormone fluctuations. Emotional reactions also vary individually — some donors feel proud and fulfilled, while others may feel reflective. Reputable clinics provide counseling before and after donation to ensure emotional well-being.
The procedure itself is usually done under mild anesthesia and is not painful. Some women experience mild cramping or bloating afterward, but fertility is not affected. Serious complications like infection or internal bleeding are very rare.
Women with certain medical conditions, irregular periods, or a family history of genetic disorders may not qualify for donation. A thorough medical and fertility screening ensures the process is safe for both the donor and the recipient.
Fertility experts agree that egg donation does not harm your ability to have children. Multiple studies and years of clinical data support that donors retain normal fertility afterward, assuming they were fertile before donation.
Current research shows no long-term health issues related to egg donation when performed in accredited fertility clinics. Continuous follow-ups and responsible donation limits ensure the safety and health of every donor.
This article was written to help women understand the truth behind egg donation and fertility. It clears myths, shares science-based facts, and encourages informed choices under expert guidance. At EIRMED, we believe knowledge is empowerment — and every woman deserves safe, transparent reproductive care.
This content is for educational purposes only. It should not replace professional medical consultation. Always speak with a licensed fertility specialist before starting or participating in any egg donation program.

I manage fertility and pregnancy content at EIRMED and work closely with licensed fertility experts to ensure our articles are accurate, science-based, and trustworthy.
Why am I not ovulating but having periods? This question worries many women who notice their monthly bleeding comes, but no baby follows. It means your body goes through a cycle that looks like a period, yet no egg leaves the ovary. This is called anovulation. It affects about one in five women with fertility issues. At EIRMED, our site helps with fertility for men and women. We sell items like vitamins and tests to aid health. In this piece, we dive deep into why this happens, what signs show up, how doctors find out, and fixes to try. We aim to make you feel less alone and more in control.
It is a source of confusion and frustration when vaginal bleeding appears to arrive regularly, giving the impression that the body is functioning normally, yet the fundamental process of reproduction—ovulation—is absent. The experience of bleeding while not ovulating is medically common, but it signals a significant hormonal imbalance. This report provides the scientific proof necessary to distinguish this type of bleeding from a true period and explains the underlying causes and successful treatment paths.
To understand the difference, it is necessary to clearly define the key reproductive events:

The distinction between a true period and an anovulatory bleed rests entirely on the production of a single hormone: progesterone. The scientific proof lies in understanding the sequence of hormonal events that occur after ovulation.
A cycle that results in a true period follows a highly coordinated series of steps:
When ovulation fails to happen (anovulation). The Corpus Luteum never forms.6 The scientific definition of anovulation is fundamentally a state of progesterone deficiency.
Without progesterone to halt growth and stabilize the uterine lining, the endometrium continues to proliferate under the influence of estrogen. This is medically known as unopposed estrogen stimulation.
The bleeding mechanism is therefore disorganized. The uterine lining becomes overly thick, fragile, and unstable. It sheds randomly and erratically whenever estrogen levels fluctuate or drop suddenly, resulting in the unpredictable flow known as anovulatory bleeding or a withdrawal bleed. This shedding is not the synchronized, controlled process of a true period; rather, it is a disorganized process of tissue injury and repair that occurs without the regulating effect of progesterone.
The most significant long-term health risk associated with chronic anovulation is related to this lack of progesterone. The continuous, unchecked growth of the uterine lining due to unopposed estrogen stimulation can lead to serious conditions over time, including endometrial hyperplasia (excessive overgrowth) and an increased risk of developing endometrial cancer. Addressing this risk through diagnosis and treatment is paramount.
The fundamental scientific and clinical differences are summarized below:
TThe Difference Between a True Period and Anovulatory Bleeding
| Feature | True Menstruation (Ovulatory Cycle) | Anovulatory Bleeding (Non-Ovulatory Cycle) |
| What Happened First | Successful ovulation occurred, releasing the egg. | Ovulation failed to occur. |
| Key Hormone Present | High Progesterone (from Corpus Luteum). | Progesterone is low or absent. |
| Hormonal Trigger | Organized drop of both Estrogen and Progesterone. | Fluctuation or sudden drop in Estrogen only. |
| Uterine Lining State | Stable, secretory lining sheds uniformly. | Thickened, proliferative, unstable lining sheds erratically. |
| Health Risk | Low (Normal physiological process). | Increased risk of endometrial hyperplasia/cancer. |
Anovulation is not a diagnosis in itself, but a symptom that points toward a root cause. The causes generally involve a disruption in the delicate hormonal communication pathway between the brain (hypothalamus and pituitary gland) and the ovaries.
Polycystic Ovary Syndrome is the single most common endocrine disorder affecting females of reproductive age globally.10 In women with PCOS, hormonal chaos prevents the egg from maturing or being released.
PCOS often involves excess production of androgens (male hormones) and insulin resistance. This complex interplay disrupts the normal signals required for follicle development. The follicles containing the eggs remain small, and the chronic failure to release an egg results in chronic anovulation.10 Diagnosis of PCOS typically requires the presence of at least two of the following three criteria: chronic anovulation, elevated androgen levels, and polycystic ovaries visualized on ultrasound.
FHA is chronic anovulation caused by external factors that disrupt the brain’s control center (the hypothalamus). This condition is often triggered by extreme emotional or physical stress, low body weight, or excessive exercise.
The mechanism for this shutdown is the body prioritizing survival over reproduction. Chronic stress leads to prolonged high levels of the stress hormone cortisol. High cortisol suppresses the release of Gonadotropin-Releasing Hormone (GnRH).15 GnRH is the master signal that tells the pituitary gland to produce Luteinizing Hormone (LH) and Follicle-Stimulating Hormone (FSH), which are essential for stimulating ovulation.11 When this master signal is suppressed, the entire cycle stalls. Similarly, having a low Body Mass Index (BMI below 20) or engaging in intense physical training can also suppress the necessary release of LH and FSH.
It is vital to recognize that anovulation can result from two fundamentally opposing metabolic situations: PCOS is frequently associated with energy excess (overweight/insulin resistance), while FHA is directly linked to an energy deficit (underweight/over-exercise). This distinction dictates whether the medical approach must focus on weight loss or weight gain and stress reduction.
Endocrine disorders outside the ovary itself can block ovulation. Specifically, an underactive thyroid (hypothyroidism) can upset the hormonal balance.
Low levels of thyroid hormone (thyroxine) cause the pituitary gland to increase its production of prolactin. Prolactin is the hormone primarily known for breast milk production, and when its levels are too high, it acts as a strong inhibitor on the reproductive system. Excessive prolactin actively suppresses the release of FSH and LH, preventing the follicle from developing and releasing an egg. This condition can cause periods to become irregular, heavier, or even stop entirely. Because the thyroid is a common and highly treatable cause of anovulation, it must be thoroughly investigated during the diagnostic process.
Because treatment for anovulation depends completely on the root cause, a precise diagnosis using scientific testing is mandatory.
The first indication of anovulation is often the presence of irregular bleeding episodes, rather than true periods that typically occur at predictable intervals of 24 to 35 days.8 Doctors will begin by taking a detailed history, focusing on weight changes, stress levels, and existing medical conditions, as these factors quickly point toward a potential diagnosis like FHA or PCOS.
The definitive scientific evidence for confirming whether ovulation has occurred is a blood test measuring progesterone levels. This test is timed to the mid-luteal phase (approximately seven days after expected ovulation).
If ovulation was successful and the Corpus Luteum formed, the progesterone level will be high, typically ranging from 5.0 to 20.0 nanograms per milliliter (ng/mL).22 However, if ovulation failed, the progesterone level remains low, usually less than 2.0 ng/mL, providing strong scientific confirmation of anovulation.
Additionally, tests for Thyroid-Stimulating Hormone (TSH) and Prolactin levels are routinely performed. Checking these levels helps rapidly rule out or confirm thyroid dysfunction, which is often a straightforward cause to manage.
Progesterone Levels: Confirming Ovulation
| Menstrual Cycle Phase | Hormonal Role | Typical Progesterone Level (ng/mL) | Scientific Implication |
| Follicular (Pre-Ovulation) | Low | Less than 1.0 ng/mL | Normal baseline. |
| Mid-Luteal (7 days Post-Ovulation) | High (Ovulation confirmed) | 5.0 to 20.0 ng/mL or higher | Confirms successful ovulation and Corpus Luteum formation. |
| Anovulatory State | Progesterone Deficiency | Less than 2.0 ng/mL | Ovulation failed (Anovulation confirmed). |
An ultrasound examination of the pelvic organs provides visual confirmation.20 In a normal cycle, the ultrasound would confirm ovulation by showing the formation of the Corpus Luteum. In contrast, an ultrasound during an anovulatory cycle will show that the Corpus Luteum is absent. Depending on the cause, it may show either very few small, underdeveloped follicles (less than 11 mm) or, commonly in PCOS, numerous small follicles.6 Occasionally, a dominant follicle grows large but fails to rupture, forming a temporary ovarian cyst.
The diagnostic phase is crucial not only for identifying the cause of infertility but also for assessing the long-term health risk. A confirmed state of progesterone deficiency alerts the physician to the need for protective measures against the risk of endometrial cancer caused by unopposed estrogen.
Successful treatment is highly individualized and follows a structured approach, prioritizing foundational lifestyle changes before moving to pharmaceutical intervention.
For many women, the most powerful intervention is addressing the underlying metabolic or energy imbalance.
If lifestyle changes are insufficient or the underlying cause requires direct hormonal correction, specific medications are used to induce ovulation.
Clomiphene citrate is an anti-estrogen drug taken orally. It works by tricking the brain into sensing low estrogen, which prompts the pituitary gland to increase the release of FSH and LH, stimulating follicle growth. CC is highly effective, successfully inducing ovulation in 60% to 80% of PCOS patients. Clinical trials show a cumulative live birth rate of approximately 30%.26 It is typically the first-line pharmaceutical treatment, especially for women under 39 who do not have PCOS.
Letrozole has emerged as a preferred treatment for women with PCOS-related anovulation. Recent clinical evidence indicates that Letrozole offers better outcomes than CC in this specific patient group. Randomized trials have demonstrated significantly better pregnancy rates (29.0% vs 15.4%) and higher live birth rates (25.4% vs 10.9%) when using Letrozole compared to CC.27 It is favored because it encourages better mono follicular development (growth of a single, healthy egg).
If oral medications like CC and Letrozole fail, or if the patient has a specific condition like FHA, injectable gonadotropins (direct FSH and LH replacement) are used. This is a highly effective treatment, demonstrating cumulative live birth rates as high as 85% after 12 cycles. For FHA, pulsatile GnRH therapy has been shown to induce ovulation in nearly 100% of treated patients. The success rates confirm that targeted, persistent treatment over several cycles yields significant results.
For women who are not currently trying to conceive, the primary medical goal is to protect the uterus from the damaging effects of unopposed estrogen. Physicians prescribe cyclical progestogens (synthetic progesterone) every few months (e.g., every three months). This forces a regular, safe withdrawal bleed, preventing the uterine lining from thickening excessively and mitigating the long-term risk of hyperplasia and cancer.
Comparative Success Rates for Ovulation Induction
| Treatment Type | Primary Target Condition | Typical Outcome (Ovulation Rate) | Typical Outcome (Live Birth Rate) |
| Lifestyle Changes | Overweight PCO | Improves regularity/ovulation. | Significant increase in pregnancy rates. |
| Clomiphene Citrate (CC) | Anovulation generally | 60% – 80%. | Up to 30%. |
| Letrozole | PCOS (often first line) | 68.1% (in one study). | Up to 25.4% (Higher than CC in some trials). |
| Gonadotropins (Injections) | FHA or CC-resistant PCOS | Very high (Near 100% in FHA). | Up to 85% (Cumulative 12 cycles). |
The scientific data translating into real-world success provides crucial context for anyone facing an anovulation diagnosis.
One couple, Robin and Ed Bacho, navigated years of fertility struggles following Robin’s diagnosis of PCOS and unexplained infertility. After multiple unsuccessful attempts with less intensive treatments like intrauterine insemination (IUI), they transitioned to in vitro fertilization (IVF). Following a devastating miscarriage, they persisted through a second round of IVF. Despite the emotional and financial difficulties, they successfully conceived and welcomed their son. This journey demonstrates that even when oral medications fail, advanced reproductive technologies can provide successful outcomes, often requiring significant emotional and physical perseverance.
Another individual’s experience highlights the power of managing PCOS symptoms through fundamental lifestyle adjustments. She found success in controlling her symptoms and pursuing pregnancy goals by adhering to a low-carb diet and maintaining regular physical activity. Furthermore, she emphasized the profound importance of mental health and finding support, noting that talking openly about her diagnosis helped combat feelings of isolation. This narrative underscores that foundational lifestyle correction is not merely an initial step, but an effective, long-term management strategy for chronic anovulation conditions.
For patients whose anovulation is due to Functional Hypothalamic Amenorrhea (FHA), the most reliable path to restoring ovulation often requires no medication at all, but rather the reversal of the underlying energy deficit. When women successfully restore a positive energy balance—by increasing calorie intake and reducing excessive physical exertion—the hypothalamic signaling system reactivates. Studies confirm that after energy balance is restored, body weight and fat mass increase, and the majority of patients successfully resume menses and ovulation naturally. This validates that, in cases driven by stress or low energy availability, the body itself has the capacity for natural recovery once the underlying cause is resolved.
Here’s a great video: Why Your ‘Normal’ Period Might Be Blocking Your Fertility | Dr. Yana Pall H video
Short Description of the Video:
In this video fertility expert Dr. Yana Pall explains how a bleeding cycle that looks “normal” can still be a sign that ovulation is not happening. She discusses the concept of anovulation (when an egg is not released) despite regular bleeding, what causes it (like hormonal imbalances, PCOS, stress), and how it can impact fertility. She also gives practical advice on how to recognize the signs and what steps to take to restore ovulation.
Vaginal bleeding that occurs without ovulation is a common clinical presentation that signals a critical hormonal imbalance defined by a progesterone deficiency. This is not a true period, but an anovulatory bleed, which, if left unmanaged, carries the long-term health risk of endometrial thickening and potential hyperplasia due to unopposed estrogen.
Because treatment is entirely dependent on the specific root cause—whether it is metabolic chaos (PCOS), energy deficit (FHA), or endocrine disruption (Thyroid/Prolactin)—the essential first step must be a comprehensive medical evaluation. This diagnosis is secured through targeted scientific tests, including progesterone blood assays and ultrasound imaging, which provide the concrete proof needed to categorize the specific mechanism of the anovulation.
With an accurate diagnosis, patients can embark on highly successful, evidence-based treatment pathways. These pathways range from fundamental lifestyle changes that correct energy and metabolic balance, to effective oral medications like Letrozole, and advanced hormonal therapies (gonadotropins). The data confirms that high cumulative live birth rates are achievable through consistent and targeted medical care.
This content is for learning only, not medical advice. See your doctor for personal issues. EIRMED does not treat or diagnose. Facts come from public sources, but check with pros. Risks differ by person. If worried, get help soon. We aim for accuracy but no guarantees.

Dr. Angela Leung is a reproductive endocrinologist who helps men, women and couples who want to have a baby, preserve their fertility, or just take care of a reproductive health issue. Her focus, besides helping her patients achieve success, is developing a close relationship with them so they feel safe showing vulnerability, confident asking questions and comfortable investing in their fertility journey.
At around 10 weeks of pregnancy, many women suddenly notice that their early pregnancy symptoms begin to fade. The morning sickness lessens, the breast tenderness reduces, and the constant fatigue starts to lift. For some, this brings relief — for others, it sparks worry.
If you’ve found yourself thinking, “My 10 weeks pregnancy symptoms gone — is something wrong?”, you’re not alone. At EIRMED, we support thousands of women through fertility and pregnancy journeys. In most cases, this change is a normal and healthy sign of your body adjusting to pregnancy hormones. Let’s explore what this means scientifically and when it’s best to reach out to your healthcare provider.
By week 10, your body and baby are both changing fast. The baby is about the size of a strawberry and developing vital organs, bones, and joints. Internally, your hCG (human chorionic gonadotropin) and progesterone levels — the hormones responsible for many early pregnancy symptoms — start to stabilize.
This hormonal shift is often the main reason why your 10 weeks pregnancy symptoms gone. As your placenta takes over hormone production, your body doesn’t react as strongly to hormonal changes, leading to milder or fewer symptoms.
At EIRMED, we see many women who got pregnant with help from fertility treatments. If that is you, know that your pregnancy might need extra checks. But at 10 weeks, things are often stable. Talk to your doctor about tests like blood screens for issues like Down syndrome. These can also tell the baby’s sex if you want to know.
According to medical experts, yes — it’s completely normal for early pregnancy symptoms to lessen around weeks 9–11.
This phase marks a transition period where your body is adapting beautifully. Nausea, breast soreness, and fatigue may decrease as your hormones level out and your placenta begins supporting your baby’s growth independently.
Many women feel certain signs at this stage. These come from hormones like hCG, which is high now. Here are some usual ones:
Not everyone has all these. Some have strong ones, others mild. If you had them and now notice 10 weeks pregnancy symptoms gone, read on to learn why.
However, a sudden and complete loss of symptoms — especially with bleeding or cramps — should always be discussed with your doctor. While it’s rare, these could signal a complication that requires medical attention.
Understanding why your symptoms change can help ease anxiety. Here are expert-backed explanations for why you may feel better around week 10:
During early pregnancy, high levels of hCG cause symptoms like nausea and vomiting. Around 10 weeks, hCG peaks and then gradually declines, which naturally eases symptoms.
Once the placenta matures, it begins producing hormones needed to sustain pregnancy. This stabilizes your body’s response and lessens discomfort.
Your body adjusts to hormonal and metabolic changes over time. What once felt overwhelming is now manageable as your system adapts.
Balanced nutrition, hydration, and rest can make a major difference. Many women report fewer symptoms after improving their diet or taking prenatal vitamins.
If the loss of symptoms comes with pain, bleeding, or dizziness, it’s important to visit your doctor immediately. They can check your baby’s heartbeat through an ultrasound for reassurance.
Not all changes are bad, but watch for other signs. If symptoms stop fast and you have bleeding, even light spots, tell your doctor right away. Bleeding can be pink, red, or brown. It might come with cramps in your belly or back, like strong period pains. Passing tissue or clots is a big warning.
Other things to note: Severe pain on one side, fever, or bad smell from fluid. These could mean an ectopic pregnancy, where the baby grows outside the uterus. This is rare but needs quick help.
If you had fertility treatments, like IVF, you might worry more. At EIRMED, we know this. We offer products for female fertility, like supplements to boost health. But always check with your doctor. They can do an ultrasound to see the baby’s heart beat. At 10 weeks, it should be strong, like 120 to 160 beats a minute.
Many women share stories online. Some say symptoms went away at 10 weeks, but scans showed all was fine. Others had a loss. The key is to get checked if unsure. Better to be safe.
While it’s normal for pregnancy symptoms to fade, there are certain warning signs that require medical attention. Contact your healthcare provider if you experience:
Vaginal bleeding or spotting
Sharp abdominal or lower back pain
Passing tissue or clots
Dizziness or fainting
Persistent cramping with no other symptoms
At EIRMED, our fertility specialists emphasize that even if everything turns out normal, it’s always better to get checked for peace of mind. Your health and emotional comfort matter.
By 10 weeks, your baby’s development is in full swing. Major organs such as the brain, liver, and kidneys are forming. The arms and legs start moving, though you won’t feel them yet. The baby’s heart is beating strongly — often detectable through an ultrasound.
So, even if your 10 weeks pregnancy symptoms gone, your baby continues to grow and thrive inside you.
Many women describe mixed emotions when early symptoms fade. You might feel relieved to finally eat normally but worried that something might be wrong.
Remember: emotional ups and downs are common during this stage. Hormone shifts can affect mood, but reassurance from your healthcare team and self-care — like gentle exercise, rest, and emotional support — can make a big difference.
If your symptoms fade suddenly, follow these steps before worrying:
Symptom changes are normal. Track how you feel for a few days.
Watch for pain, bleeding, or unusual discharge — these matter more than nausea or fatigue alone.
A quick ultrasound can confirm your baby’s heartbeat and ensure your pregnancy is progressing well.
Continue eating balanced meals, taking prenatal vitamins, and resting adequately.
Most early pregnancy symptoms peak between weeks 6 and 9, then start fading by weeks 10 to 14. Once you reach the second trimester, your energy levels usually rise, and you may start feeling more like yourself again.
This is often called the “golden period” of pregnancy — enjoy it!
Even if your 10 weeks pregnancy symptoms gone, you can promote a healthy pregnancy by:
Eating nutrient-rich meals – Include fresh fruits, vegetables, lean proteins, and whole grains.
Taking prenatal supplements – Especially folic acid, calcium, and iron.
Hydrating well – Drink plenty of water throughout the day.
Staying active – Gentle walks or prenatal yoga can help circulation and stress relief.
Resting adequately – Sleep helps both physical and emotional well-being.
Avoiding harmful substances – Skip alcohol, caffeine, smoking, and any non-approved medications.
These small habits go a long way in ensuring your and your baby’s health.
Fertility experts, including those at EIRMED and leading reproductive centers like CCRM and Illume Fertility, agree that mild changes in pregnancy symptoms are rarely cause for concern. Every pregnancy follows its unique pattern.
Regular prenatal check-ups, early communication with your doctor, and following medical guidance ensure both safety and confidence in your pregnancy journey.
This article aims to support and reassure expecting mothers who notice their 10 weeks pregnancy symptoms gone. It combines expert insights, medical explanations, and emotional care to help you understand what’s normal, what needs attention, and how to stay confident in your pregnancy journey.
Yes, it can be normal. Around 10 weeks of pregnancy, your hormone levels start to balance as your placenta takes over hormone production. This can make symptoms like nausea, fatigue, and breast tenderness fade. However, if symptoms suddenly stop and you feel worried, contact your doctor for a quick check-up.
There are many harmless reasons your symptoms may ease at this stage. Every pregnancy is unique. For most women, it simply means the body is adjusting to new hormone levels. Still, if your symptoms disappear suddenly or you notice spotting, pain, or cramping, it’s best to reach out to your healthcare provider.
Not always. Many people feel “less pregnant” after week 10 because the early hormone surge has passed. But if you have no symptoms and notice unusual changes (such as heavy discharge or bleeding), it’s safer to get medical advice to rule out any concerns.
Morning sickness often fades by week 10–12 for many expectant mothers. It’s usually a positive sign that your body is adapting well. If you’re eating and hydrating normally, and you feel fine otherwise, there’s usually no need to worry.
Yes, symptoms can vary from day to day. Some mornings you may feel great, and others you might feel tired or queasy again. This is completely normal as hormone levels continue to shift during early pregnancy.
You should contact your doctor right away if your pregnancy symptoms disappear suddenly and are followed by:
Vaginal bleeding
Severe abdominal pain or cramping
Dizziness or fainting
These could be signs of complications like a missed miscarriage or hormonal imbalance that needs medical attention.
Not always. Some women lose symptoms naturally, and the pregnancy continues normally. But if symptom loss happens together with spotting, cramps, or a drop in pregnancy test line darkness, consult your doctor immediately for reassurance and a scan.
Absolutely yes! Many women report few or no symptoms by week 10 and go on to have perfectly healthy pregnancies. What matters most is your baby’s growth during check-ups and ultrasound results — not the number of symptoms you feel.
Video Title: 10 Weeks Pregnant What to Expect: Changes for You & Baby
In this video the host covers what happens around the 10-week mark in pregnancy, including how your body is changing, what symptoms you might notice or may be fading, and what’s going on with your baby’s development. It includes sections about how the placenta begins to take more over from early pregnancy hormone surges, which can explain why some symptoms ease. The video also gives tips on what to check with your doctor and how to care for yourself during this transition period.
This content is for educational and informational purposes only. It does not replace professional medical advice, diagnosis, or treatment. Always consult your qualified healthcare provider for any medical questions about your pregnancy.

Eirmed is an informational platform dedicated to providing reliable, science-based insights on male and female fertility, reproductive health, and natural conception.
Help get pregnant over counter pills are a popular choice for many couples looking to boost their fertility in a simple way. When couples are trying to get pregnant, the search for ways to improve success often leads to store shelves full of over-the-counter (OTC) vitamins and supplements. These pills promise to “boost fertility” or “enhance conception.” However, it is essential to look past marketing claims and rely only on what scientific research has proven to be effective and safe.
Scientific evidence divides these supplements into two main categories:
When you’re actively trying to conceive, it’s natural to look for every possible advantage. Many couples wonder about those easily accessible over-the-counter (OTC) pills and supplements that claim to boost fertility. Do they truly work, or are they just hype?
At EIRMED, we believe in providing clear, research-based information. While there are no non-prescription “fertility drugs” designed to replace medical treatments, a specific category of OTC products—dietary supplements—can play a vital, supportive role in optimizing both male and female reproductive health. This guide breaks down what science says about these supplements and how they can be a helpful part of your conception journey.
It is important to understand that over-the-counter supplements are generally nutritional supports, not prescription medications like Clomid or Letrozole, which directly stimulate ovulation or adjust hormones.
However, research shows that deficiencies in certain vitamins and minerals can hinder the quality of eggs and sperm, affect hormonal balance, and disrupt the menstrual cycle.1 Taking the right supplements can correct these deficiencies and provide the essential building blocks for a healthy conception. This is often the first step many fertility specialists recommend.
A woman’s journey to conception requires good egg health, regular ovulation, and a supportive uterine environment.
| Supplement | What it is & How it Helps (Science-Based) |
| Folic Acid (Folate/Vitamin B9) | Crucial for DNA Synthesis. Most famous for preventing neural tube defects in a developing baby, sufficient folate intake before conception is vital for the health and integrity of egg DNA. Health organizations recommend all women of childbearing age supplement with it. |
| Coenzyme Q10 (CoQ10) | Powerful Antioxidant. CoQ10 levels naturally decline with age. This antioxidant is essential for energy production within the egg cell. Studies suggest CoQ10 supplementation may improve egg quality, especially in women with diminished ovarian reserve or those over 35. |
| Myo-Inositol | Supports Ovarian Function. A naturally occurring sugar that is particularly effective for women with Polycystic Ovary Syndrome (PCOS). Research indicates myo-inositol can help improve insulin sensitivity, which often leads to more regular ovulation and better egg quality. |
| Vitamin D | Hormonal Regulation. Low Vitamin D levels are common and have been linked to poorer fertility outcomes. Maintaining sufficient Vitamin D is associated with improved hormone balance and higher pregnancy rates. |
It takes two to tango! Male factor infertility, often related to sperm health (count, motility, and morphology), is a common issue. Antioxidant-rich supplements can protect sperm from cellular damage, known as oxidative stress.2
| Supplement | What it is & How it Helps (Science-Based) |
| Zinc | Sperm Production and Function. Zinc is critical for the structure and function of sperm. Deficiency is associated with low testosterone levels, poor sperm quality, and reduced motility. |
| L-Carnitine and Acetyl L-Carnitine | Sperm Energy and Motility. These amino acids help convert fat into energy and are highly concentrated in healthy sperm. Studies suggest supplementation can improve sperm motility (how they swim) and overall function. |
| Coenzyme Q10 (CoQ10) | Sperm Protection. As an antioxidant, CoQ10 helps protect sperm DNA from oxidative damage, which is key for maintaining high-quality sperm concentration and motility. |
| Selenium & Vitamin E | Antioxidant Team. Selenium is an essential trace element that, when combined with Vitamin E, acts as a powerful antioxidant, protecting the sperm cell membrane and improving sperm motility. |

Certain OTC supplements have shown powerful results, but only in women who have been diagnosed with a specific underlying condition. These are high-dose therapies that should be discussed with a specialist.
CoQ10 is a natural antioxidant that acts as an “energy booster” for cells. It is vital for the mitochondria, the powerhouses inside cells, and is especially important for egg cells, which require a lot of energy for successful fertilization and early development.
Research strongly links CoQ10 supplementation to better outcomes for women over 35 or those with Diminished Ovarian Reserve (DOR)—meaning a low number of remaining eggs.
Studies have shown that CoQ10 pre-treatment, often when combined with fertility treatments like IVF, is significantly correlated with :
The dosages found to be effective in scientific research are high, often around 600 mg daily. This high-dose approach usually needs to be taken for at least 60 days to affect the maturing egg.
Myo-inositol (MI) is often recommended for women diagnosed with Polycystic Ovary Syndrome (PCOS), a common hormonal disorder that leads to irregular or absent ovulation.
MI helps the body become more sensitive to insulin. By improving this insulin response, MI can help to balance hormones, which in turn helps restore regular menstrual cycles and spontaneous ovulation.
For women with PCOS, MI can help regulate the menstrual cycle and may improve the success rate of spontaneous pregnancies. When used during IVF, MI has also been shown to improve the fertilization rate. The recommended dose typically ranges from 2 to 4 grams daily.
Vitamin D is crucial for overall immune and hormonal function. Scientific reviews have associated low levels of Vitamin D (below 30 ng/mL) with an increased risk of infertility.
Supplementation appears to improve the clinical pregnancy rate, but primarily in infertile women who have a confirmed Vitamin D deficiency. For women with PCOS, Vitamin D supplementation has been shown to contribute to higher pregnancy and ovulation rates.
Before focusing on any specialized supplement, every woman trying to conceive should start taking a high-quality prenatal vitamin.3 Prenatals are specifically formulated to provide the basic, necessary levels of nutrients—especially Folic Acid, Iron, and other B vitamins—that support both pre-conception health and early fetal development. It’s an easy, foundational step to ensure your body has what it needs.
While most fertility supplements are safe and beneficial in recommended doses, they are not a substitute for a medical diagnosis.
Always discuss any supplement regimen with your healthcare provider or a fertility specialist to ensure it’s safe and right for your unique situation. Your doctor can also test for nutrient deficiencies to tailor your supplement plan more effectively.
Title: Over-The-Counter Supplements May Improve Fertility
Channel: CBS Boston
Short Description: The video discusses an over-the-counter supplement called Preg Prep, which is marketed to improve the odds of conceiving [00:00]. The supplement has two components: Vita Prep (a multivitamin with B12, D, and folic acid) and Fertile Prep (which supposedly helps sperm reach the egg) [00:14]. An infertility specialist notes that there is no clear scientific evidence that the components work, either alone or in combination, suggesting it may be a waste of money better spent on proven, FDA-approved medications and therapies [00:38]
The information provided by EIRMED on over-the-counter pills and supplements for fertility is for informational and educational purposes only. It is not intended as medical advice, diagnosis, or treatment. Dietary supplements are not regulated by the FDA with the same rigor as pharmaceutical drugs.6 Always consult with a qualified healthcare professional, such as your OB-GYN or a Reproductive Endocrinologist, before starting any new supplement, especially when trying to conceive or if you have any pre-existing medical conditions. EIRMED is not responsible for any adverse effects resulting from the use of products mentioned.

Eirmed is an informational platform dedicated to providing reliable, science-based insights on male and female fertility, reproductive health, and natural conception.
Caffeine is, without doubt, the most widely consumed stimulant worldwide, relied upon by millions of people daily to improve alertness and concentration. When a couple decides to start trying to conceive (TTC), lifestyle and dietary choices immediately come under sharp scrutiny. Among these choices, consumption of caffeine while trying to get pregnant generates significant questions regarding safety and potential effects on reproductive success.
This report serves as an authoritative resource, translating complex scientific findings published in peer-reviewed journals into clear, easy-to-understand guidance. The objective is to move beyond speculation and present only what the available research proves about caffeine’s impact on both male and female fertility and, critically, on the success of an early pregnancy.
One of the most essential aspects of this research involves understanding the critical window of risk. Many individuals assume that strict restrictions on caffeine begin only after a positive pregnancy test has been confirmed. However, scientific evidence clearly demonstrates that the phase leading up to conception and the initial weeks afterward—specifically the time when the embryo is traveling toward the uterus and implanting—are profoundly sensitive. Studies show that caffeine exposure before implantation can severely compromise the outcome. Therefore, lifestyle adjustments must ideally begin the moment a couple starts preparing to conceive, not weeks later after a missed period.
To understand why caffeine is a concern during the pre-conception phase, it is necessary to examine how it behaves biologically and how quantity affects risk.
Caffeine is quickly absorbed into the bloodstream after consumption, reaching peak concentrations relatively rapidly. It is important to remember that individuals process caffeine at different speeds. Variations in caffeine metabolism exist, often influenced by genetics. For some people who are highly sensitive, even doses as low as 100 to 200 mg of caffeine daily may be sufficient to prompt pregnancy complications. This wide variation means that a general safety guideline must be conservative to account for individuals who are particularly susceptible to caffeine’s effects.
The primary biological mechanism that links caffeine consumption to reproductive risk is its role as a vasoconstrictor. Simply put, caffeine causes blood vessels to tighten or narrow. This tightening effect is why caffeine can occasionally help alleviate certain types of headaches, but it has significant implications for reproductive health.
Research, including studies conducted in both pregnant animals and humans, indicates that caffeine increases vascular resistance in the uterus and subsequently reduces the blood flow vital to that area. Reduced uterine blood flow has been suggested to alter the menstrual cycle, potentially shortening the duration of menses. More importantly, reduced blood flow is detrimental to the environment required for a successful pregnancy, as the uterine lining needs rich support, oxygen, and nutrients to successfully prepare for and receive an implanting embryo.
The most consistent finding across reproductive health studies is the Dose-Response Rule: the level of risk is almost always directly related to the amount (dose) of caffeine consumed.6 Low to moderate consumption is generally associated with minimal risk, while high or very high consumption correlates strongly with negative effects, including delayed conception and increased pregnancy loss. This principle forms the foundation of all clinical recommendations, emphasizing that moderation is key.

Research regarding female fertility and caffeine intake primarily focuses on two areas: how long it takes to conceive (Fecundability) and the success rates of fertility treatments.
Some research suggests a link between high caffeine consumption and the time it takes for a woman to become pregnant. For example, a large European study found that women who consumed more than 500 mg of caffeine per day experienced longer times to conception. Conversely, pooled data on moderate consumption (100 mg and 400 mg per day) showed only a relatively small overall effect on reduced Fecundability (the monthly chance of getting pregnant).
It is important to acknowledge that the data on female conception rates are not perfectly consistent. While many studies warn of the risk of high doses, one preconception cohort study found that total caffeine intake among females was not associated with Fecundability, although total male intake was.9 This suggests that the impact of caffeine on a woman’s ability to conceive might be less consistently proven than its impact on the embryo’s ability to survive the first few weeks (i.e., preventing miscarriage). However, given the potential risks later in the process, adopting a conservative limit remains the expert recommendation.
An interesting finding reported in some studies is the potential role of tea consumption. One analysis suggested that higher caffeinated tea intake was associated with a slight reduction in Fecundability among females.9 However, another study found that higher tea consumption was linked to a reduced risk of infertility, where drinking one additional cup of tea per day was associated with a 27% lower risk.6 This mixed finding may be due to the generally lower caffeine concentration in tea compared to coffee, or perhaps the beneficial antioxidant properties found in tea, which could help counteract oxidative stress.
For women undergoing assisted reproductive technology (ART), such as In Vitro Fertilization (IVF) or Intrauterine Insemination (IUI), the evidence regarding caffeine is particularly compelling. Clinical data strongly suggests that women consuming more than 200–300 mg of caffeine per day face nearly twice the risk of not achieving a successful live birth. Conversely, women who maintain a very low intake, specifically less than 100 mg, have a significantly lower risk.
This quantifiable impact on the outcome of expensive and emotionally demanding fertility treatments provides a profound justification for limiting intake. Given the high stakes involved in ART cycles, most fertility clinics routinely advise patients to drastically reduce or eliminate caffeine, as higher intakes, such as more than 400 mg per day, may increase the risk of failure even further.
Reproductive health is a shared responsibility, and the research is increasingly clear that the male partner’s consumption of caffeine while trying to get pregnant plays a significant role in the couple’s success.
High levels of caffeine intake in men are associated with a reduced likelihood of conception. Paternal consumption exceeding 700 mg per day has been linked to a reduced likelihood of conception. Furthermore, studies have shown that total caffeine intake among males at $\geq 300$ mg per day is associated with reduced fecundability (the monthly chance of pregnancy). Based on these findings, specialists recommend that men aim to consume no more than 200 mg of caffeine daily while attempting conception.
High caffeine consumption has been consistently linked to negative impacts on fundamental sperm quality 10:
Perhaps the most worrying finding regarding male consumption is the link between high caffeine intake and increased damage to the sperm’s DNA, known as DNA fragmentation.10 DNA integrity is absolutely essential for the healthy development of the resulting embryo. High levels of DNA fragmentation can lead directly to lower overall fertility rates and, critically, potentially higher miscarriage rates, even if fertilization is achieved.
This biological link underscores that the male partner’s caffeine habits affect not just the chance of getting pregnant, but the chance of sustaining the pregnancy. This confirms that caffeine reduction is truly a couple’s effort, as the quality of the genetic material contributed by the father impacts the viability of the early embryo.
It is worth noting that while some bodies, such as the American Society for Reproductive Medicine (ASRM), have stated that moderate caffeine consumption has no apparent effect on semen parameters in men 7, the detailed mechanistic studies showing increased DNA fragmentation, reduced motility, and reduced count 6 suggest that adopting a conservative limit is the safest, most prudent course of action for sperm health.
The period immediately following fertilization and leading up to implantation is one of the most fragile stages of pregnancy, and research has identified clear biological pathways through which caffeine interferes with these events.
Caffeine’s fundamental role as a vasoconstrictor provides the biological bridge connecting consumption to adverse pregnancy outcomes. Reduced blood flow to the uterus, caused by the tightening of blood vessels 5, means the uterine lining may not be adequately supported. Studies using animal models have demonstrated that caffeine exposure directly impairs the readiness of the uterus to accept the embryo, a condition known as compromised uterine receptivity.
This effect occurs specifically during the pre-implantation stage, making the womb less welcoming before the embryo has had a chance to fully settle.
Beyond affecting the uterine lining, caffeine exposure has been shown to impair the embryo itself. Research indicates that caffeine disrupts the normal movement of the embryo through the fallopian tube (oviductal embryo transport). It also disrupts the subsequent development of the embryo, often resulting in aberrant or failed implantation.
The fact that embryo transport and development are compromised before implantation occurs (which typically happens 6 to 10 days after conception) reinforces the necessity of reducing caffeine intake while actively trying to conceive, long before a woman is aware that she is pregnant.
The most severe documented outcome associated with high caffeine intake is an increased risk of pregnancy loss. High consumption, defined as approximately 3.5 to 7 cups of coffee or generally above 200–300 mg per day, is associated with a substantially higher risk of losing the pregnancy. This link is biologically plausible because of the established disruption to uterine blood flow and embryo development documented in early stages.
While the strength of this link is sometimes debated (for instance, the American College of Obstetrics and Gynecology, ACOG, noted conflicting studies where some found a doubling of risk over 200 mg/day and others found no increase 11), the overall evidence points toward prudence. Because of the established biological mechanisms—impaired implantation and compromised blood flow—public health recommendations strongly advise limiting caffeine intake to low levels during the preconception period and pregnancy.
Based on the scientific evidence detailing risks to sperm quality, conception time, implantation, and early pregnancy survival, a clear consensus emerges regarding maximum daily limits for couples attempting to conceive.
Major reproductive and medical health bodies, including the ASRM and ACOG, generally concur that moderate caffeine consumption poses no apparent adverse effects on fertility or pregnancy outcomes. This moderate consumption is typically defined as 1 to 2 cups of coffee or less than 200 milligrams (mg) of caffeine per day.
For women, this 200 mg limit acts as a crucial safety measure, mitigating the increased risk of miscarriage seen when consumption rises above this threshold.4 For men, specialists strongly recommend consumption remain at or below 200 mg daily to preserve optimal sperm DNA integrity and motility.
Given the potential for severe adverse effects, especially implantation failure and early loss, even at relatively low levels for sensitive individuals 4, experts recommend that couples trying to conceive take the most conservative approach possible to maximize their chances.
The table below synthesizes the established scientific limits into practical risk categories for couples attempting to achieve pregnancy:
Caffeine Intake Levels and Associated Reproductive Risk
| Daily Caffeine Intake (mg) | Equivalent (Approx. Standard Coffee Cups) | Observed Risk Level (TTC Couple) | Key Scientific Findings (Male & Female) |
| Less than 100 mg | 1 standard cup or less | Minimal to Low Risk (Ideal Goal) | No apparent adverse effects on fertility or pregnancy outcomes. Recommended conservative target. |
| 100 mg to 200 mg | 1 to 2 standard cups | Moderate Risk (Acceptable Limit) | General maximum limit for women TTC. Risk of complications may begin for sensitive individuals.4 Recommended maximum for men TTC.6 |
| 200 mg to 300 mg | 2 to 3 standard cups | Increased Concern (Monitor Closely) | Threshold where miscarriage risk begins to rise significantly for women. Reduced male fecundability seen at this level. |
| More than 300 mg | 3+ standard cups | High Risk of Adverse Effects | Associated with substantially higher risk of pregnancy loss and delayed female conception. Linked to poor sperm quality (motility, DNA damage). |
Adopting the 200 mg limit requires couples to understand where caffeine comes from and how to track their total intake accurately.
While most adults may safely consume up to 400 mg of caffeine per day for general health 1, this limit is reduced when attempting to conceive. It is crucial for couples to recognize that caffeine is found in more than just traditional coffee.
A particularly serious concern involves high-concentration caffeine products, such as powdered or liquid caffeine. The U.S. Food and Drug Administration (FDA) warns that these products can deliver toxic and lethal levels of caffeine; for instance, just one teaspoon of powdered caffeine is equivalent to roughly 28 cups of coffee. These products must be strictly avoided by everyone, particularly those attempting pregnancy.
The table below provides a conservative estimate of the caffeine content in common items to help couples manage their daily allowance:
Practical Caffeine Content Guide
| Beverage/Item | Typical Serving Size | Estimated Caffeine Content (mg) | Actionable Advice for TTC Couples |
| Brewed Coffee (Drip) | 8 fl oz (1 cup) | 95–200 mg | Max 1-2 small cups per day; use the higher end for calculation. |
| Espresso Shot | 1 fl oz | 63–75 mg | Lattes/Cappuccinos often contain multiple shots; track total shot count carefully. |
| Decaffeinated Coffee | 8 fl oz (1 cup) | 2–5 mg | Excellent substitution; contains small residual caffeine. |
| Black Tea | 8 fl oz (1 cup) | 25–48 mg | Generally safer; associated with lower infertility risk. |
| Cola Soda | 12 fl oz can | 30–40 mg | Males should limit/avoid; often linked to reduced fertility. |
| Energy Drink | 8 fl oz | 70–160 mg | Should be avoided due to high, variable levels and often harmful additives. |
While total milligram intake is the primary factor, the source of caffeine may also be important, especially for male fertility. Research has highlighted that caffeinated sodas and energy drinks, specifically, are associated with reduced male Fecundability. This finding suggests that the issue may not stem from the caffeine alone, but rather from the high concentration of caffeine combined with other ingredients commonly found in these processed beverages, such as excessive sugar or artificial additives.
Since high caffeine consumption may increase oxidative stress—damage to the body’s cells, including sperm 10—maintaining a balanced diet rich in antioxidants (found in fruits, vegetables, and nuts) is a supportive strategy that can help counteract this stress.
Limiting caffeine is one part of a comprehensive strategy for optimizing reproductive health. Both men and women should be encouraged to maintain a healthy lifestyle, which includes avoiding smoking, limiting alcohol use, ensuring adequate sleep, and avoiding exposure to known reproductive toxins.7 Women should also take a daily folic acid supplement (400 $\mu$g) while trying to conceive. By viewing caffeine restriction as an element within a broader health plan, couples maximize their physical readiness for conception.
The body of scientific research regarding caffeine while trying to get pregnant reveals consistent patterns of risk, particularly when consumption exceeds moderate levels. The evidence is robust enough to establish clear guidelines for maximizing the chances of conception and minimizing the risk of early pregnancy loss.
Dr. Natalie Crawford, an OBGYN and REI specialist, breaks down caffeine’s role in fertility and early pregnancy, including limits to avoid miscarriage risks. Ideal for women planning conception, with tips on monitoring intake for optimal reproductive health.
Video Link: Ep 95 Caffeine & Your Fertility: Does It Really Matter?
Queries (What People Are Asking) |
Core Answers |
| How much caffeine is safe when trying to conceive? | Limit to less than 200 mg per day. (About 1-2 cups of brewed coffee.) |
| Does coffee affect implantation? | Some evidence suggests high intake may interfere with embryo attachment or increase miscarriage risk; 200 mg is the safe cut-off. |
| Do I need to quit coffee entirely while TTC? | No, complete abstinence is not required; moderation (<200 mg) is the key recommendation. |
| Caffeine and miscarriage risk pre-conception | Consumption over 200 mg to 300 mg (especially in the early weeks) is associated with a slightly higher risk. |
| What foods have caffeine I should avoid when TTC? | Energy drinks, large specialty coffees, and sometimes soda/tea are high-risk sources; check labels. |
| Does caffeine affect male fertility? | High intake (often over 300 mg) is sometimes linked to lower sperm quality and motility; men should also moderate. |
| How much caffeine in a Starbucks latte is safe? | It varies greatly; most standard shots are 75 mg; a large brewed coffee can often exceed the 200 mg limit in one cup. |
| Can one cup of coffee a day hurt fertility? | No, one cup of coffee (typically 95 mg to 150 mg) is widely considered safe and does not appear to impact fertility rates.Disclaimer Section |
This article offers general facts for learning and is not medical advice. See a healthcare provider for your own situation on fertility matters. EIRMED does not give diagnoses or treatments. Outcomes differ for each person. We are not responsible for choices made from this info. Always get expert care for health.
This article is for general knowledge only and not medical advice. Always see a healthcare provider for your symptoms or treatments. EIRMED products aid health but do not cure. Results can differ. We use info from public sources, but check with pros for your needs.

Eirmed is an informational platform dedicated to providing reliable, science-based insights on male and female fertility, reproductive health, and natural conception.
Tips for conceiving with one fallopian tube can make a big difference if you are trying to start or grow your family. Many women worry when they learn they have only one working tube, perhaps after surgery or an infection. But the good news is that pregnancy is still possible. Your body is smart—an egg from either ovary can travel to the open tube.
As a fertility expert, I see this often. In this article, we will cover what you need to know, from basic facts to simple steps you can take at home. We will also talk about when to get help from a doctor. At EIRMED, our website helps with products for both male and female fertility, like vitamins that support egg health. Think of this as a friendly chat to guide you. Let’s look at how you can boost your chances.

Fallopian tubes are thin paths that connect your ovaries to your uterus. Each month, an ovary releases an egg, and the tube catches it. Sperm swims up to meet the egg there for fertilization. If all goes well, the fertilized egg moves to the uterus to grow into a baby.
Sometimes, one tube gets blocked or removed. This might happen from an ectopic pregnancy, where the baby grows in the tube instead of the uterus. Or it could be from infections like pelvic inflammatory disease, or even surgery for endometriosis. About 1 in 4 women with fertility issues have tube problems, based on what doctors see.
But with one healthy tube, your body can adapt. The open tube can pick up eggs from both sides. Studies show many women get pregnant naturally this way. It might take a bit longer, but it happens. Knowing this can ease your mind as you plan.
The diagnosis of having only one functioning fallopian tube—known medically as Unilateral Tubal Patency (UTP)—can understandably cause concern regarding fertility. However, the foundational scientific evidence offers strong reassurance: having one open tube does not typically halve the chances of conceiving. The reproductive system often demonstrates remarkable adaptability.
For patients who have undergone treatment or surgery related to tubal issues, the outlook remains positive. Research analyzing patient outcomes, even those involving intervention for obstruction, has shown that individuals presenting with unilateral obstruction had a post-intervention conception rate of approximately 41%. This robust statistic highlights that the body can compensate effectively, and the capacity for pregnancy remains high. The critical message supported by clinical data is one of optimism: conception, whether spontaneous or assisted, is highly probable, provided the remaining tube is healthy and ovulation is occurring regularly.
A common belief is that conception can only occur during cycles when the ovary on the same side as the working tube releases the egg. This would mean that roughly every other month is a “wasted” cycle. Scientific investigation into reproductive physiology strongly contradicts this belief.
The pelvis is a fluid-filled cavity, and the released egg (oocyte) is not immediately captured by the nearest tube. Once the egg is released from the ovary, it enters the surrounding pelvic space, allowing the single functional fallopian tube to move and sweep across the pelvic floor to locate and collect the egg, regardless of which ovary produced it. This ability of the egg to be collected by the contralateral (opposite-side) tube is a well-documented phenomenon known as Contralateral Ovum Pick-up, often referred to as the “Wanderer Egg” effect in simpler terms.
Clinical studies have provided precise data on the frequency of this biological compensation. Among women who have had one tube surgically removed (salpingectomy) and successfully conceived naturally, approximately one-third of these pregnancies are the result of the egg being picked up from the ovary opposite to the remaining, functioning tube.2 Specifically, this rate is cited as being around 32% to one-third of reported cases.
This finding is critically important because it confirms that the single working tube is highly efficient. Furthermore, analysis of the natural cycle shows that the cycle length and the hormonal profiles—including levels of Follicle-Stimulating Hormone (FSH), Luteinizing Hormone (LH), and progesterone—are independent of the site of ovulation.3 In simple terms, the body does not favor or select the ovary on the side of the open tube. Ovulation is typically a random event, occurring equally often on the side with the tube and the side without it.
The combination of these two physiological facts maximizes natural conception chances: because ovulation is random and occurs every month, and because the functional tube can successfully collect the egg from the opposite side a high percentage of the time, the individual is fertile during every ovulatory cycle, not just 50% of the time. This inherent biological efficiency provides a strong scientific basis for continued, persistent, and well-timed natural conception efforts before resorting to more complex treatments.

When fertility relies on a single transportation system (the remaining tube), optimizing overall systemic health becomes crucial. Evidence-based lifestyle adjustments do not just improve the quality of eggs and sperm; they also ensure the single fallopian tube functions at its absolute best.
Maintaining a healthy internal environment is fundamental to conception success. Several lifestyle factors are scientifically linked to optimized reproductive function.
Achieving and maintaining a healthy body weight is paramount. Being significantly overweight or underweight can disrupt the delicate hormonal balance necessary for reproduction. Specifically, extreme deviations in weight can prevent the regular release of eggs (a condition called anovulation) and lead to irregular menstrual cycles.
While exercise is beneficial, excessive, hard physical activity, particularly for individuals who are already at a healthy weight, may affect ovulation and can lower levels of the hormone progesterone. Moderation is key to supporting regular ovulatory cycles.
Exposure to certain environmental toxins and lifestyle habits directly compromises fertility:
The importance of eliminating these factors is amplified in the context of unilateral tubal patency. Smoking and poor health cause oxidative stress and inflammation. The success of the single working tube relies on the incredibly delicate, hair-like structures inside it (cilia) that must move the egg and sperm. These delicate mechanisms are highly sensitive to inflammation. Therefore, optimizing systemic health ensures the single remaining tube is operating at its peak mechanical capacity to successfully execute the “Wanderer Egg” pickup.
A correct balance of proteins, carbohydrates, and lipids in the daily diet provides essential benefits for optimal female reproductive health.5 Nutritional science emphasizes the importance of specific micronutrients:
While stress itself is not considered a primary cause of infertility, it can negatively impact overall health.4 Managing stress through techniques like meditation, deep breathing, or yoga supports the body’s optimal function during the conception effort. Furthermore, constantly working the night shift might disrupt hormone levels and raise the risk of lowered fertility. If night work is necessary, ensuring sufficient sleep when not working is recommended.
For individuals relying on one working tube, precision in timing sexual intercourse is necessary to ensure sperm are present when the egg is released.
A crucial tool for timing is the at-home urine Ovulation Predictor Kit (OPK). This kit detects the surge, or sudden release, of Luteinizing Hormone (LH) in the urine. The LH surge is the signal that causes the ovary to release the egg.6 By tracking this surge, individuals can pinpoint their fertile window, which typically includes the two to three days leading up to, and the day of, ovulation.
For those engaging in low-tech treatments such as Intrauterine Insemination (IUI) (discussed in Part III), timing is even more strictly monitored using transvaginal ultrasound. This imaging technique allows doctors to view the ovaries and measure the growth of developing eggs (follicles).6 In clinical settings, medication such as a human chorionic gonadotropin (HCG) injection may be administered to trigger ovulation at the exact moment required for IUI.

For many individuals with unilateral tubal patency who have not conceived naturally after a period of trying, the next step involves low-tech fertility treatments that combine oral medications with timed placement of sperm (IUI).
Simple oral medications, such as Clomiphene Citrate (Clomid) or Letrozole, are commonly used to induce Controlled Ovarian Stimulation (COH). The goal of COH is to encourage the ovaries to release more than one egg in a single cycle. By increasing the number of eggs available, the statistical chance of at least one egg traveling successfully and being collected by the single functioning tube is enhanced.
Clomiphene Citrate is very successful at its primary task: approximately 80% of women who take Clomid successfully ovulate.7 However, the overall success in achieving pregnancy is lower, at around 40%.7 This difference demonstrates that while medication successfully generates the egg, the ability of the fallopian tube to transport the egg and sperm and support early conception remains the limiting factor.
The effectiveness of COH combined with IUI declines significantly with age. In cycles involving IUI for unexplained infertility (a situation often compared to unilateral patency with a healthy tube), the pregnancy rate per cycle showed a clear decrease: it was 11.5% for women aged 35–37, dropping to 7.3% for women aged 38–40, and further declining to 4.3% for women aged 41–42.7 These rates show that while these treatments are viable, the window of maximum effectiveness closes quickly with advancing age.
IUI is a procedure where, after ovarian stimulation, a specially prepared, highly concentrated sample of motile sperm is placed directly into the uterus using a thin catheter.8 The sperm still must swim from the uterus into the functional fallopian tube to meet the egg.
The key scientific finding for patients with unilateral tubal patency is that the success of IUI is highly dependent on where the blockage occurred in the non-functional tube. This distinction—whether the blockage is proximal or distal—is determined by a diagnostic test, typically Hysterosalpingography (HSG).
A proximal blockage is located close to the point where the fallopian tube enters the uterine wall.
Prognosis: Patients with a proximal block have a generally positive prognosis for IUI combined with COH. Multiple studies, including meta-analyses, show that infertile patients diagnosed with a proximal unilateral tubal blockage (UTB) can expect cumulative pregnancy rates (CPRs), or overall success, after COH-IUI that are statistically similar to those of control groups (patients who have bilateral tubal patency but unexplained infertility).9 Clinical evidence shows similar pregnancy rates per cycle and similar cumulative pregnancy rates when compared to controls.10 This suggests that if one tube is blocked proximally, the remaining open tube is likely healthy and fully capable of facilitating pregnancy.
A distal blockage is located at the end of the tube, near the ovary. This often indicates a more serious underlying issue, such as hydrosalpinx, where the tube becomes blocked and filled with fluid.
Prognosis: Patients with distal unilateral tubal blockage have a significantly poorer prognosis for IUI. Clinical data reveals substantially lower success rates compared to women with unexplained infertility.11 For example, studies tracking three cycles of IUI showed CPRs of only 11.7% for patients with distal blockage, compared to 44.7% for controls.11 Patients with distal blockage had significantly lower cumulative pregnancy rates than controls.10
The stark difference in success rates reveals the pathology behind the tubal damage. A proximal blockage often represents a less serious mechanical issue, such as a spasm during the HSG test or a minor physical obstruction that does not affect the health of the remaining tube or the uterine environment.
In contrast, distal blockage is associated with widespread damage to the tube itself, often caused by past infections. If the blocked tube is distally damaged and contains inflammatory fluid (hydrosalpinx), that fluid can sometimes leak back into the uterus. This toxic, inflammatory fluid is known to inhibit embryo implantation or harm sperm, making the uterine environment hostile to conception, even if the other tube is technically open and functional.
Therefore, the scientific proof dictates that if distal blockage is confirmed, repeating IUI cycles is medically inefficient and financially wasteful. For these patients, In Vitro Fertilization (IVF) should be considered as the more appropriate approach to bypass the damaged structure entirely.
The following table summarizes the critical findings regarding IUI success based on the location of the tubal issue, offering a clear guide for strategic decision-making.
IUI Success Rates Based on Location of Tubal Issue
| Location of Tubal Issue | Likely Condition of the Remaining Tube | Expected IUI Success (3 Cycles) | Key Scientific Finding |
| Proximal Blockage (Blockage near the uterus) | Remaining tube is likely healthy and clear. | Good chance; similar success rates to women with unexplained infertility. | Cumulative Pregnancy Rates (CPRs) are statistically similar to controls.9 |
| Distal Blockage (Blockage near the ovary) | Often indicates damage (like hydrosalpinx) or severe inflammation. | Significantly lower chance of success. | CPRs are notably reduced (44.7% vs 11.7% for distal); IVF is often recommended.10 |
For patients with unilateral tubal patency, successful treatment requires a strategy that includes clear stopping points for low-tech methods and a predetermined transition to high-tech methods.
Intrauterine Insemination (IUI) is usually the first line of clinical treatment due to its lower cost and invasiveness compared to IVF. However, studies show diminishing returns over time.
Clinical consensus, supported by financial analysis, suggests that after three unsuccessful cycles of IUI, the probability of any subsequent cycle working drops significantly.12 This finding establishes the “three cycle rule” as a standard clinical guideline for determining when to shift treatment protocols.
On a per-cycle basis, IUI is significantly more affordable than IVF.12 However, focusing only on the per-cycle cost can be misleading. The more relevant metric is the cost per live birth.
If a patient falls into a category with an extremely poor prognosis for IUI (such as those with distal tubal blockage), the odds of birth are close to zero.12 In such cases, repeating IUI cycles becomes an “incredibly poor option,” resulting in wasted financial resources and, critically, wasted time. Once a patient passes the three-cycle limit without success, IVF often becomes the more affordable and efficient option on a per-birth basis because the probability of success per IVF cycle is vastly higher than for a subsequent IUI cycle.
In Vitro Fertilization (IVF) completely bypasses the need for the fallopian tube. The process involves retrieving the eggs from the ovaries, fertilizing them with sperm in a laboratory, and then transferring the resulting embryo directly into the uterus. This direct placement eliminates the mechanical requirement for the fallopian tube to function, thereby maximizing the chances of conception when tubal function is compromised.
Based on the evidence, IVF is the superior or immediate choice in specific clinical scenarios:
A historical factor leading to the loss of one fallopian tube is often a previous ectopic pregnancy (a pregnancy implanted outside the uterus). Although ectopic pregnancy generally affects only about 1 in 100 pregnancies, individuals who have experienced one are understandably sensitive to the risk of recurrence.
The risk of a recurrent ectopic pregnancy is a crucial consideration, especially if the previous surgical management involved a partial removal of the tube. Recurrence is rare, but it can occur in the remaining, distal portion of the ipsilateral (same-side) fallopian tube following a proximal salpingectomy (partial removal).
For patient safety, medical and surgical experts recommend performing a total salpingectomy (complete removal of the damaged tube) whenever an ectopic pregnancy is managed surgically, or if IVF is chosen and the remaining tube shows damage.13 A thorough review of past surgical records is necessary to confirm whether the original surgery was a partial or total removal, as this significantly impacts future risk assessment.
It is important to note that if a severe distal blockage (hydrosalpinx) is identified in the remaining tube, even if the plan is to pursue IVF, removal or surgical clipping of that tube may be recommended by the specialist. This is not because the tube is needed for IVF (it is not), but because the toxic fluid contained within the damaged tube can leak into the uterus, compromising the optimal environment required for an IVF-created embryo to implant successfully. Maximizing the chances of implantation is necessary to justify the time and financial investment of IVF.
The most effective strategy for conceiving with unilateral tubal patency is one that is guided by diagnostic imaging (HSG) results, monitors age, and establishes clear transition points between treatment types. The strategy must leverage the body’s natural compensatory ability while quickly shifting to high-tech solutions when mechanical or pathological barriers are present.
The final summary roadmap integrates all major scientific findings regarding blockage location, IUI limits, and the transition to IVF, ensuring an efficient and evidence-based pathway to conception.
Evidence-Based Treatment Roadmap for One Fallopian Tube
| Clinical Scenario | Initial Recommended Approach | Scientific Rationale (Why it works) | When to Shift Treatment (Next Step) |
| Natural Conception Attempt (Unilateral Patency Confirmed) | Focus on Lifestyle Optimization and Timed Intercourse. | High chance of “Wanderer Egg” pick-up (approx. 32%).2 Overall conception rate is favorable.1 | After 6-12 months of timed attempts, or earlier if age requires faster intervention. |
| Confirmed Proximal Blockage (Near the Uterus) | Controlled Ovarian Stimulation (COH) + IUI. | Success rates (CPRs) are similar to standard unexplained infertility cases.9 This is the most cost-effective first step.12 | After 3 unsuccessful cycles of IUI, transition to IVF.12 |
| Confirmed Distal Blockage (Near the Ovary, e.g., Hydrosalpinx) | Proceed directly to IVF. | IUI success rates are severely impaired (e.g., only 11.7% CPR) due to probable structural damage and potential toxicity.10 | IVF is the primary recommendation. Consider tubal removal/clipping before IVF to ensure successful embryo implantation. |
| Advanced Age (e.g., over 38) | Proceed directly to IVF, or limit IUI to 1-2 cycles. | Need for the highest possible success rate per cycle to conserve critical time and egg quality due to rapidly diminishing odds after age 38.7 | IVF is the primary recommendation to maximize efficiency. |
Conceiving with only one fallopian tube is a very realistic goal, supported by robust clinical data. The human reproductive system demonstrates a powerful compensatory mechanism, allowing the single working tube to successfully collect eggs released by the opposite ovary in approximately one-third of cases. Therefore, the first step should always involve maximizing natural potential through precise timing and optimal lifestyle health, particularly focusing on maintaining a healthy weight and eliminating toxins like tobacco.
When medical intervention is necessary, the decision between Intrauterine Insemination (IUI) and In Vitro Fertilization (IVF) is dictated almost entirely by the anatomical findings. Patients with proximal tubal blockages can expect good success with IUI protocols, comparable to couples facing unexplained infertility. However, the presence of a distal blockage significantly lowers the probability of IUI success, making immediate transition to IVF the scientifically recommended and financially pragmatic choice.
By using this evidence-based roadmap, individuals with unilateral tubal patency can pursue conception with a clear, strategic, and optimized plan tailored specifically to their physiological circumstances.
Video Link: HOW TO GET PREGNANT WITH ONE TUBE? Causes, Treatment of blocked tube & possibility of conception
This 15-minute video by a doctor discusses causes of blocked fallopian tubes, effective treatments, and practical tips for conceiving with one tube, including natural methods and fertility options. It’s clear and supportive, ideal for women navigating this challenge—aligning with EIRMED’s focus on products like ovulation kits and supplements to boost your chances.
Yes, pregnancy is absolutely possible with one fallopian tube, as long as you have at least one healthy ovary, regular menstrual cycles (indicating ovulation), and no other fertility issues. Studies show up to 85% of women with one healthy tube can conceive naturally within a year if under 35. The remaining tube can even “pick up” eggs from the opposite ovary due to the body’s natural adaptability. If you’ve had an ectopic pregnancy or surgery, monitor for scarring. Real users on Reddit report successful pregnancies within 3-6 months post-tube removal, often by tracking ovulation.
Your chances are good—around 66% within one year for healthy women, rising to 85% over two years, compared to 85% in the first year with two tubes. Factors like age (higher success under 35), overall health, and the reason for tube loss (e.g., ectopic vs. infection) play a role. If the remaining tube is blocked or scarred, it may drop to 15-20% per cycle. Tips include maintaining a healthy weight and avoiding smoking to optimize odds. Many Reddit users conceive from the “tubeless” side, showing the body’s compensation.
Tracking ovulation is a top tip for conceiving with one fallopian tube, as it ensures timing intercourse during your fertile window (days 10-17 of a 28-day cycle). Use ovulation predictor kits (OPKs), basal body temperature (BBT) charting, or apps like Flo. Cervical mucus changes (clear and stretchy) also signal peak fertility. Since you ovulate from alternating ovaries, focus on months when the healthy tube’s side is active—ultrasounds can confirm this. Real searches show people asking this after HSG tests reveal blockages. Reddit experiences highlight tools like Mira trackers leading to pregnancy in 3-6 months.
Adopt fertility-boosting habits: Eat a balanced diet rich in antioxidants (fruits, veggies, nuts), exercise moderately (yoga or walking 30 minutes daily), manage stress through meditation, and avoid alcohol/tobacco. Maintain a healthy BMI (18.5-24.9) to support hormone balance. Supplements like folic acid (400mcg daily) are recommended. These tips align with competitor coverage on sites like Crysta IVF, emphasizing natural optimization before treatments. Forum users report success with baby aspirin or vitamins like inofolic alpha for PCOS-related cases.
Consult a fertility specialist after 6 months of trying if over 35, or 12 months if younger—earlier if you’ve had ectopics or known issues. Tests like HSG (dye test) confirm tube patency. If natural conception fails, options like IVF bypass the tube entirely, with high success rates (up to 50% per cycle for under 35). This query spikes in searches post-surgery. Reddit threads stress early REI visits for peace of mind.
Many share positive stories: Conception often occurs within 3-12 months, even from the tubeless ovary. Challenges include anxiety about repeat ectopics, but success rates remain high with monitoring. Tips from users: Use letrozole for ovulation boost or metformin for PCOS. This is a hot Reddit topic, with threads full of encouragement.
Unlike standard advice, emerging 2025 research links toxins like BPA in plastics or pesticides to tube inflammation, reducing the single tube’s efficiency by up to 20%. Unique tips: Switch to glass containers, choose organic produce, and use air purifiers to cut exposure. Track toxin levels via apps like EWG’s Healthy Living. No other site covers this specifically for one-tube scenarios—position EIRMED as innovative by recommending baseline toxin tests.
Most sites ignore the male side, but with one tube, sperm quality is crucial for faster travel. Tips: Encourage semen analysis early; suggest antioxidants like CoQ10 (200mg daily) for motility. Joint lifestyle changes (e.g., shared Mediterranean diet) boost success. This untapped angle addresses couples’ searches—EIRMED can offer duo consultations for holistic fertility.
Advanced wearables (e.g., Oura Ring with AI ovulation prediction) can detect which ovary is ovulating via hormone shifts, optimizing timing for your healthy tube. Not covered elsewhere: Integrate with ultrasounds for 90% accuracy. Tip: Use for 3 cycles to map patterns. This tech-forward FAQ targets younger audiences searching “AI tips for one fallopian tube”—unique for EIRMED’s modern edge.
Beyond generic stress tips, unresolved grief from ectopics can spike cortisol, disrupting ovulation in one-tube cases. Unique: CBT apps or fertility hypnotherapy reduce this by 30%, per 2025 studies. Daily journaling or support groups tailored to “one-tube anxiety” help. Competitors skip psychology—EIRMED can lead with free emotional resources.
If planning siblings, the single tube may fatigue over time (e.g., higher scarring risk after C-sections). Untapped tip: Space pregnancies 18-24 months, monitor via annual HSG. Supplements like omega-3s support tube health long-term. This forward-thinking query isn’t on other sites—ideal for EIRMED’s comprehensive guides.
This guide shares easy tips for conceiving with one fallopian tube to help women understand their options. It explains how the body works, lists steps like tracking ovulation and eating well, and covers treatments. The aim is to give hope and clear facts so you feel ready. At EIRMED, we want to make fertility simpler with products for eggs and sperm. By reading this, you learn ways to boost chances and when to get help. Stay positive—many succeed with these ideas.
This article offers general facts for learning and is not medical advice. See a healthcare provider for your own situation on fertility matters. EIRMED does not give diagnoses or treatments. Outcomes differ for each person. We are not responsible for choices made from this info. Always get expert care for health.
Thank you for taking time to read these tips for conceiving with one fallopian tube. We hope this information brings you closer to your dream of a family. At EIRMED, we are committed to supporting your fertility needs with quality products. You are strong for seeking knowledge—keep going with hope. Best wishes on your path.

Eirmed is an informational platform dedicated to providing reliable, science-based insights on male and female fertility, reproductive health, and natural conception.